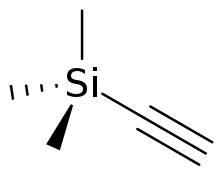
Trimethylsilylacetylene
Trimethylsilylacetylene is a colorless liquid that is a derivative of acetylene. Called "tms acetylene", it is used as a source of "HC2−". The trimethylsilyl group can then be cleaved off with TBAF. Using this protected alkyne, as opposed to acetylene itself, prevents further coupling reactions and also has the benefit of being a liquid.[1] A less expensive alternative reagent is 2-methylbut-3-yn-2-ol, which after alkynylation is deprotected with base.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethynyltri(methyl)silane | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | TMSA |
| ChemSpider | |
| ECHA InfoCard | 100.012.655 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10Si | |
| Molar mass | 98.220 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.69 g/mL |
| Boiling point | 53 °C (127 °F; 326 K) |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H225, H315, H318, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Trimethylsilylacetylene is commercially available. It may also be prepared in a manner similar to other silyl compounds: deprotonation of acetylene with a Grignard reagent, followed by reaction with trimethylsilyl chloride.[2]
Trimethylsilylacetylene is a precursor to 1,4-bis(trimethylsilyl)buta-1,3-diyne, a protected form of 1,3-butadiyne.[3]
References
- Godson C. Nwokogu, Saskia Zemolka, Florian Dehme (2007). "Trimethylsilylacetylene". EROS. doi:10.1002/047084289X.rt288.pub2. ISBN 978-0471936237.
{{cite encyclopedia}}: CS1 maint: uses authors parameter (link) - Andrew B. Holmes and Chris N. Sporikou (1993). "Trimethylsilylacetylene". Organic Syntheses.; Collective Volume, vol. 8, p. 606
- Graham E. Jones, David A. Kendrick, and Andrew B. Holmes (1987). "1,4-Bis(trimethylsilyl)buta-1,3-diyne". Organic Syntheses. 65: 52. doi:10.15227/orgsyn.065.0052.
{{cite journal}}: CS1 maint: multiple names: authors list (link)