Tetrahydroharmol
Tetrahydroharmol is a bioactive beta-carboline harmala alkaloid.[1] It acts as a reversible inhibitor of monoamine oxidase A.[2]
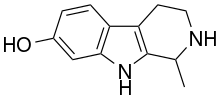 | |
| Clinical data | |
|---|---|
| Other names | 1,2-Dihydroharmaline |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H14N2O |
| Molar mass | 202.257 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Legal Status
Australia
Harmala alkaloids are considered Schedule 9 prohibited substances under the Poisons Standard (October 2015).[3] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[3]
See also
References
- Riba, J; McIlhenny, EH; Valle, M; Bouso, JC; Barker, SA (2012). "Metabolism and disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca". Drug Testing and Analysis. 4 (7–8): 610–6. doi:10.1002/dta.1344. PMID 22514127.
- Buckholtz, Neil S.; Boggan, William O. (1 November 1977). "Monoamine oxidase inhibition in brain and liver produced by β-carbolines: structure-activity relationships and substrate specificity". Biochemical pharmacology. Elsevier BV. 26 (21): 1991–1996. doi:10.1016/0006-2952(77)90007-7. ISSN 0006-2952. PMID 921812.
- Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.