Simufilam
Simufilam is an investigational drug candidate for the treatment of Alzheimer's disease.[1][2] Developed by an American firm Cassava Sciences (previously Pain Therapeutics), based in Austin, Texas, it is a small molecule that was reported to restore impaired function of key neuronal receptors and improve cognition in mice. Preclinical data also show reduced tau hyperphosphorylation, reduced neuroinflammation and improved synaptic plasticity (by activity-dependent arc expression). Lindsay Burns (Senior Vice President, Neuroscience) and Hoau-Yan Wang of the City University of New York (science advisor to the company) are the main scientists who led the research on the discovery and development of the drug.
 | |
| Clinical data | |
|---|---|
| Other names | PTI-125, PTI-910 |
| Routes of administration | Oral administration (tablets) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 4.5 hrs[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
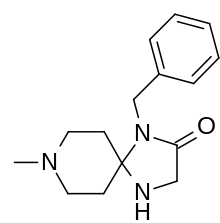
| Formula | C15H21N3O |
| Molar mass | 259.353 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Promising results from a first-in-patient open-label clinical trial were reported in 2020 in 13 Alzheimer subjects. Biomarkers from cerebrospinal fluid that are associated with Alzheimer's (for the disease itself, as well as neurodegeneration and inflammation) were significantly reduced from baseline after 28-day treatment.[1] A subsequent randomized, placebo-controlled clinical trial showed similar improvements from baseline in CSF biomarkers and significant differences from placebo. Simufilam gained attention following presentation of interim cognitive data from an open-label (no placebo) extension study that showed a mean improvement from baseline in the first 50 Alzheimer's disease subjects to complete 6, 9 and 12 months of treatment. After a surge in stock price, short sellers of Cassava Sciences stock filed a citizen petition with the Food and Drug Administration in August 2021 to stop the clinical trials based on alleged research misconduct, which focused primarily on Western blot images from mostly unrelated publications as old as 2005. FDA has denied the petition, and no journal found evidence of data manipulation, despite highly-publicizedretractions of five papers in PLOS One in early 2022 that are all unrelated to Alzheimer's disease, with two involving the filamin A target of simufilam for its role in regulating opioid receptors. The Journal of Neuroscience and Neurobiology of Aging stated they found no evidence of data manipulation, but issued expressions of concern to acknowledge the ongoing institutional investigation.
History
From research funded by Cassava Sciences (then Pain Therapeutics), Lindsay Burns (later Senior Vice President, Neuroscience) with Hoau-Yan Wang and Maya Frankfurt of the City University of New York, reported in PLOS One the binding of a 300-kDa protein with naloxone to prevent drug dependence.[3] This report, the authors claimed, was a critical discovery of the high-affinity binding site of certain opioid antagonists (naloxone and naltrexone) to a cytoskeletal protein called filamin A (FLNA), a critical molecule in maintaining cell shape and division.[4] Burns and Wang reported the pentapeptide binding region the next year.[5] In 2010, they identified a new compound which they described as "a novel analgesic" and named PTI-609 (PTI for Pain Therapeutics, Inc.) that could also bind FLNA.[6] In 2012, their research team characterized a different novel compound (PTI-125) from their proprietary medicinal chemistry library as a potential therapeutic drug for Alzheimer's disease in The Journal of Neuroscience. Their report showed PTI-125 similarly binding to FLNA as naloxone and naltrexone. They claimed that this binding was of medical relevance in Alzheimer's disease because filamin A's interaction with the alpha7 nicotinic acetylcholine receptor was critical for Abeta42 signaling through this receptor to hyperphosphorylate tau.[4][7] Their report in Neurobiology of Aging in 2017 in 2017 showed an experiment in Alzheimer mice that PTI-125 reduced tau hyperphosphorylation and inflammatory cytokine release and improved function of alpha7 nicotinic acetylcholine receptors, NMDA receptors and insulin receptors.[8][9]
In 2018, the National Institutes of Health granted the company a research award that totaled $3.9 million with supplements for the Phase 2 clinical trials of PTI-125 in mild-to-moderate Alzheimer's disease subjects.[10][11] Once fully focused on Alzheimer's disease, the company was renamed Cassava Sciences, Inc. |url=https://www.globenewswire.com/news-release/2019/03/27/1773930/8339/en/Pain-Therapeutics-Announces-Name-Change-to-Cassava-Sciences-Inc.html |access-date=2022-04-28 |website=GlobeNewswire |language=en}}</ref> In August 2020, the United States Adopted Names (USAN) assigned the drug chemical name as simufilam, and Barbier announced it as "an absolutely new type of drug therapy for Alzheimer’s disease."[12] In 2020, it was announced that the initial biomarker analysis of the second clinical trial had failed due to high variability in CSF biomarker changes seen even in placebo subjects over this 28-day trial. It was later determined that this initial biomarker analysis showed no correlation between biomarkers in change from baseline (mean pearson's r=0.06), which is unexpected because biomarkers generally move together. This lack of correlation was especially evident in individual placebo subjects' data that showed huge improvements in one biomarker coincident with worsening in another. Back-up CSF samples were analyzed at a different lab and showed good results, confirming results from the earlier Phase 2a trial[13] to which Barbier declared: "the first drug—to our knowledge—that can restore cognition."[14]
The final stage, Phase III trials started in October 2021. The first phase would require 750 participants, and the second, 1000.[15] As of April 2022, 60 people were enrolled in the study with an additional 170 in screening.
Pharmacology and controversies
Simufilam disrupts two critical pathogenic pathways in Alzheimer's disease by disrupting filamin A's linkage to the alpha7 nicotinic acetylcholine receptor and to toll-like receptor 4. Controversy exists around whether simufilam restores an altered conformation of filamin A, because both the altered conformation and its restoration to normal by simufilam have been demonstrated by one technique: shifts in isoelectric focusing point of filamin A in diseased versus normal tissue and after treating with simufilam. Although other researchers have evaluated simufilam in other disease models, e.g. Angelique Bordey of Yale, all the knowledge on FLNA binding by simufilam comes from Burns' and Wang's research.[16] The original discovery paper claimed that FLNA is the binding site of certain opioid antagonists in reducing and preventing drug dependence;[3] and likewise simufilam.[4] They showed that simufilam binds FLNA and reduces levels of complexes of amyloid beta 42 and the alpha-7 nicotinic receptor.[1][7] These complexes are hypothesized to have cytotoxic effects on neurons and the soluble Abeta42 is considered a causative agent in Alzheimer's disease.[17][18] Preventing or lowering the amounts of soluble Abeta42 bound to this and other receptors is believed to be a key focus in Alzheimer drug development.[19] Because simufilam reduces tau hyperphosphorylation by preventing or disrupting filamin A's linkage to the alpha7 nicotinic acetylcholine receptor and to toll-like receptor 4, Burns and Wang hypothesized that blocking these pathogenic pathways by dissociating filamin A would slow the progression of Alzheimer's disease.[7]
No other researchers have examined simufilam binding to FLNA[7] or simufilam's restoration of altered filamin A.[8][9] [20][21] The papers that reported the discovery of FLNA binding by naloxone or naltrexone were recently retracted for "unresolved issues" relating to "similarities in background pixels" of Western blot images, but with no evidence of data manipulation.[22][23]
The link between FLNA and Alzheimer's disease is novel and will be ultimately determined by clinical data.
Allegations of Research Issues by Short Seller Petitioners
In August 2021, Jordan A. Thomas of the law firm Labaton Sucharow in New York City, submitted a citizen petition to the Food and Drug Administration to investigate simufilam research.[14] The petitioners' allegations "[raise] grave concerns about the quality and integrity of the laboratory-based studies surrounding this drug candidate and supporting the claims for its efficacy,"[24] and made four key points:
- Claims that filamin A is associated with Alzheimer's disease and that simufilam binds to filamin A are not supported by any independent research;
- Images of western blots in Burns and Wang's papers that described simufilam' activities showed anomalies and data manipulations;
- The biomarker data in clinical trials also indicate possible data manipulations; and
- "Remarkable" molecular experiments that looked suspicious.[25]
Thomas expressed that "The volume of problematic material uncovered in publicly available sources indicates a thorough audit would likely unveil significant additional scientific misconduct and data manipulation," and that "the methodology allegedly used in these experiments defies logic."[26] Cassava Sciences publicly defended against each claim asserting that "the claims made in this post [petition] regarding scientific integrity are false and misleading. The Company stands behind its science, its scientists and its scientific collaborators, and is responding to ensure the facts are known and respected."[27] Barbier maintained that the research data were genuine, and said, "As a science company, we champion facts that can be evaluated and verified."[26]
Cassava Sciences further stated: "Cassava Sciences' plasma p-tau data from Alzheimer's patients was generated by Quanterix Corp., an independent company, and presented at the recent Alzheimer’s Association International Conference."[27] Quanterix immediately made a public disclaimer that although Quanterix conducted the sample analysis, it was not involved in analyzing the data (because they remain blind to treatment group) stating: "Quanterix or its employees did not interpret the test results or prepare the data charts presented by Cassava."[28][29]
In November 2021, David Bredt, a former neuroscientists at Johnson & Johnson,[30] who mysteriously left venture capital firm MPM after a 4-month stint in August when the petition was filed, was revealed as the lead whistleblower in the The Wall Street Journal.[31] Early in 2021, Bredt became aware of the drug as the shares of Cassava Sciences reached a notable height. Bredt was at J&J when Cassava Sciences (then Pain Therapeutics) presented their Alzheimer's disease program under CDA to J&J. Examining the clinical trials, he was concerned with the lack of placebos.[14] Further scrutiny of all the research papers made him conclude that, "They were making statements that were incompatible with biology and with pharmacology," and such important discoveries -- if born out by Phase 3 clinical trial data -- deserved to "win five Nobel Prizes." Teaming up with a childhood friend Geoffrey Pitt, a cardiologist and a professor at Weill Cornell Medical College, he directed Thomas for the petition.[14]
The petition was denid by Patrizia Cavazzoni, Director of the Center for Drug Evaluation and Research, in February 2022.[32] The letter concluded:
We take the issues you raise seriously. Please note that your Petitions are being denied solely on the grounds that your requests are not the appropriate subject of a citizen petition. This response does not represent a decision by the Agency to take or refrain from taking any action relating to the subject matter of your Petitions.[33]
Reactions from the journals
The 2012 paper in The Journal of Neuroscience was the one which reported that simufilam could bind to filamin A,[7] the key claim for the potential use of the drug in Alzheimer's disease. The study relies on the western blot data, of which representative images are published. Marina R. Picciotto, the editor-in-chief, reported in November 2021 that although she found "no evidence of data manipulation," there was one erroneous duplication of a brain image in a panel of several.[31] The journal published an erratum correcting this error and also publishing original uncropped Western blot images (that had been questioned) in December 2021. The journal declared that the "error does not affect the conclusions of the article."[34] However, the journal received further PubPeer complaints that the original images created further questions, and it issued an expression of concern on 19 January 2022 to acknowledge the ongoing institutional investigation:
The editors have been made aware of concerns about Western blots in this study, including those published with the article's erratum (Wang et al., 2021[35]). These and other concerns are currently under investigation by the academic authorities at the City University of New York (CUNY). JNeurosci will await the outcome of that investigation before taking further action.[20]
PLOS One re-examined all Burns and Wang's research papers and came to conclusion in March 2020 that there were "unresolved issues" relating to "similarities in background pixels" in the Western blot images; they did not find evidence of data manipulation. It retracted five of Wang's papers, two of which were co-authored with Burns,[36] which examined the protein target of simufilam in the context of regulating opioid receptors.[4] The retraction note on the original discovery paper[3] states:
The data and comments provided did not resolve the concerns about the integrity and reliability of data presented in this article. In light of these issues, the PLOS ONE Editors retract this article. HYW [Hoau-Yan Wang] and LB [Lindsay Burns] did not agree with the retraction. MF [Maya Frankfurt] either did not respond directly or could not be reached. HYW stands by the article’s findings.[22]
Neurobiology of Aging, Neurobiology of Aging, which published the 2017 paper reporting the ability of simufilam to restore altered filamin A in mice, in response to PubPeer complaints, examined the paper [37]and stated "the editors did not find compelling evidence of data manipulation intended to misrepresent the results" but noted errors to be corrected in a corrigendum. It issued an expression of concern while awaiting the outcome of the institutional investigation: "The authors have requested a corrigendum to correct these issues. However, Neurobiology of Aging is aware of an ongoing inquiry of these and other concerns by the sponsoring institution, the City University of New York (CUNY), and will make a final decision as to appropriate corrective action once that inquiry has been concluded."
References
- Wang HY, Pei Z, Lee KC, Lopez-Brignoni E, Nikolov B, Crowley CA, et al. (2020). "PTI-125 Reduces Biomarkers of Alzheimer's Disease in Patients". The Journal of Prevention of Alzheimer's Disease. 7 (4): 256–264. doi:10.14283/jpad.2020.6. PMID 32920628. S2CID 211039039.
- Clinical trial number NCT04388254 for "Simufilam (PTI-125), 100 mg, for Mild-to-moderate Alzheimer's Disease" at ClinicalTrials.gov
- Wang, Hoau-Yan; Frankfurt, Maya; Burns, Lindsay H. (2008-02-06). "High-affinity naloxone binding to filamin a prevents mu opioid receptor-Gs coupling underlying opioid tolerance and dependence". PLOS One. 3 (2): e1554. doi:10.1371/journal.pone.0001554. PMC 2212716. PMID 18253501.
- Burns, Lindsay H.; Wang, Hoau-Yan (2017). "Altered filamin A enables amyloid beta-induced tau hyperphosphorylation and neuroinflammation in Alzheimer's disease". Neuroimmunology and Neuroinflammation. 4 (12): 263–271. doi:10.20517/2347-8659.2017.50. PMC 8294116. PMID 34295950.
- Wang, Hoau-Yan; Burns, Lindsay H. (2009). "Naloxone's pentapeptide binding site on filamin A blocks Mu opioid receptor-Gs coupling and CREB activation of acute morphine". PLOS One. 4 (1): e4282. doi:10.1371/journal.pone.0004282. ISSN 1932-6203. PMC 2628740. PMID 19172190.
- Burns, Lindsay H.; Wang, Hoau-Yan. "PTI-609: A Novel Analgesic that Binds Filamin A to Control Opioid Signaling". Recent Patents on CNS Drug Discovery (Discontinued). 5 (3): 210–220. doi:10.2174/157488910793362386. PMID 20726836.
- Wang, H.-Y.; Bakshi, K.; Frankfurt, M.; Stucky, A.; Goberdhan, M.; Shah, S. M.; Burns, L. H. (2012). "Reducing Amyloid-Related Alzheimer's Disease Pathogenesis by a Small Molecule Targeting Filamin A". Journal of Neuroscience. 32 (29): 9773–9784. doi:10.1523/JNEUROSCI.0354-12.2012. PMC 6621293. PMID 22815492.
- Wang, Hoau-Yan; Lee, Kuo-Chieh; Pei, Zhe; Khan, Amber; Bakshi, Kalindi; Burns, Lindsay H. (2017). "PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer's disease pathogenesis". Neurobiology of Aging. 55: 99–114. doi:10.1016/j.neurobiolaging.2017.03.016. PMID 28438486.
- Toniolo, Sofia; Sen, Arjune; Husain, Masud (2020). "Modulation of Brain Hyperexcitability: Potential New Therapeutic Approaches in Alzheimer's Disease". International Journal of Molecular Sciences. 21 (23): 9318. doi:10.3390/ijms21239318. PMC 7730926. PMID 33297460.
- "Multiple Ascending Dose clinical trial of PTI-125, a novel AD therapeutic candidate". nih.gov. 2018. Retrieved 2022-04-29.
- Cassava Sciences, Inc. (2021-09-07). "A Phase 2b, Randomized, Double-blind, Placebo-controlled, Multiple Dose, Biomarker and Safety Study of PTI-125 in Mild-to-moderate Alzheimer's Disease Patients". National Institute on Aging (NIA).
- Inc, Cassava Sciences (2020-08-24). "Cassava Sciences Announces Lead Drug Candidate PTI-125 Is Assigned the Chemical Drug Name 'sumifilam' by USAN". GlobeNewswire News Room. Retrieved 2022-05-03.
- Wang, H.-Y.; Pei, Z.; Lee, K.-C.; Lopez-Brignoni, E.; Nikolov, B.; Crowley, C.A.; Marsman, M.R.; Barbier, R.; Friedmann, N.; Burns, L.H. (2020). "PTI-125 Reduces Biomarkers of Alzheimer's Disease in Patients". The Journal of Prevention of Alzheimer's Disease: 1–9. doi:10.14283/jpad.2020.6.
- Keefe, Patrick Radden (2022-01-15). "Jordan Thomas's Army of Whistle-Blowers". The New Yorker. Retrieved 2022-04-29.
- Inc, Cassava Sciences (2021-12-23). "Cassava Sciences Launches Clinical Website to Support Phase 3 Studies of Oral Simufilam in Alzheimer's Disease". GlobeNewswire News Room. Retrieved 2022-04-30.
- Mandavilli, Apoorva (2022-04-18). "Scientists Question Data Behind an Experimental Alzheimer's Drug". The New York Times. ISSN 0362-4331. Retrieved 2022-04-28.
- Dineley, Kelly T. (2007). "Beta-amyloid peptide--nicotinic acetylcholine receptor interaction: the two faces of health and disease". Frontiers in Bioscience. 12: 5030–5038. doi:10.2741/2445. PMID 17569627.
- Buckingham, Steven D.; Jones, Andrew K.; Brown, Laurence A.; Sattelle, David B. (2009). "Nicotinic acetylcholine receptor signalling: roles in Alzheimer's disease and amyloid neuroprotection". Pharmacological Reviews. 61 (1): 39–61. doi:10.1124/pr.108.000562. PMC 2830120. PMID 19293145.
- D'Andrea, Michael R.; Nagele, Robert G. (2006). "Targeting the alpha 7 nicotinic acetylcholine receptor to reduce amyloid accumulation in Alzheimer's disease pyramidal neurons". Current Pharmaceutical Design. 12 (6): 677–684. doi:10.2174/138161206775474224. PMID 16472157.
- "Expression of Concern: Wang et al., "Reducing Amyloid-Related Alzheimer's Disease Pathogenesis by a Small Molecule Targeting Filamin A"". Journal of Neuroscience. 42 (3): 529–529. 2022-01-19. doi:10.1523/JNEUROSCI.2306-21.2021. ISSN 0270-6474. PMID 34921050.
- "Expression of Concern: Wang et al., (2017) PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer's disease pathogenesis. Neurobiol. Aging, 55:99-114". Neurobiology of Aging. 113: 152. 2022. doi:10.1016/j.neurobiolaging.2022.03.012.
- "Retraction: High-Affinity Naloxone Binding to Filamin A Prevents Mu Opioid Receptor–Gs Coupling Underlying Opioid Tolerance and Dependence". PLOS One. 17 (3): e0266627. 2022-03-30. doi:10.1371/journal.pone.0266627. ISSN 1932-6203. PMC 8967022. PMID 35353861.
- "Retraction: Naloxone's Pentapeptide Binding Site on Filamin A Blocks Mu Opioid Receptor–Gs Coupling and CREB Activation of Acute Morphine". PLOS One. 17 (3): e0266629. 2022. doi:10.1371/journal.pone.0266629. PMC 8967007. PMID 35353864.
- "Important Deadline Reminder: Kessler Topaz Meltzer & Check, LLP Reminds Cassava Sciences, Inc. Investors of Securities Fraud Class Action Lawsuit". www.law.com. 2021-10-28. Retrieved 2022-05-01.
- Thomas, J.A. (2021-08-23) [2021-08-18]. "Citizen Petition from Labaton Sucharow". www.regulations.gov. Retrieved 2022-04-29.
- McKenzie, Heather (2021-08-27). "UPDATED: Cassava Sciences Responds to Allegations that Data is Manipulated and "Defies Logic"". BioSpace. Retrieved 2022-04-28.
- Inc, Cassava Sciences (2021-08-25). "Cassava Sciences Responds to Allegations". GlobeNewswire News Room. Retrieved 2022-05-01.
- "Quanterix Releases Statement". Quanterix. 2021-08-27. Retrieved 2022-04-29.
- "Quanterix Releases Statement". www.businesswire.com. 2021-08-27. Retrieved 2022-04-29.
- Rockoff, Jonathan D. (2011-03-03). "J&J Poaches Lilly's Neurological Research Chief". Wall Street Journal. ISSN 0099-9660. Retrieved 2022-04-29.
- Michaels, Dave; Walker, Joseph (2021-11-17). "SEC Investigating Cassava Sciences, Developer of Experimental Alzheimer's Drug". Wall Street Journal. ISSN 0099-9660. Retrieved 2022-04-29.
- "On Procedural Grounds, FDA Denies Citizen Petition to Look Into Data Tampering at Cassava Sciences". www.fdanews.com. 2022-02-14. Retrieved 2022-05-01.
- Cavazzoni, P. (2022-02-10). "Response Letter from FDA CDER to Labaton Sucharow". www.regulations.gov. Retrieved 2022-04-29.
- "Erratum: Wang et al., "Reducing Amyloid-Related Alzheimer's Disease Pathogenesis by a Small Molecule Targeting Filamin A"". The Journal of Neuroscience. 41 (50): 10405–10405. 2021-12-15. doi:10.1523/JNEUROSCI.2154-21.2021. ISSN 0270-6474.
- "Erratum: Wang et al., "Reducing Amyloid-Related Alzheimer's Disease Pathogenesis by a Small Molecule Targeting Filamin A"". Journal of Neuroscience. 41 (50): 10405–10405. 2021-12-15. doi:10.1523/JNEUROSCI.2154-21.2021. ISSN 0270-6474. PMC 8672690. PMID 34759033.
- Akst, Jef (2022-03-31). "PLOS ONE Pulls Five Papers Tied to Alzheimer's Drug Controversy". The Scientist. Retrieved 2022-04-28.
- Wang, Hoau-Yan; Lee, Kuo-Chieh; Pei, Zhe; Khan, Amber; Bakshi, Kalindi; Burns, Lindsay H. (2017). "PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer's disease pathogenesis". Neurobiology of Aging. 55: 99–114. doi:10.1016/j.neurobiolaging.2017.03.016.