Sabizabulin
 | |
| Names | |
|---|---|
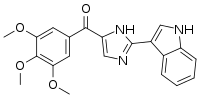
| IUPAC name
[2-(1H-Indol-3-yl)-1H-imidazol-5-yl]-(3,4,5-trimethoxyphenyl)methanone | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C21H19N3O4 | |
| Molar mass | 377.400 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sabizabulin is a chemical compound from the group of indole and imidazole derivatives. It is being studied as a mitotic inhibitor and chemotherapeutic agent in castration-resistant metastatic prostate cancer[4] and in SARS-CoV-2 (COVID-19) infections.[5]
Properties
Sabizabulin is orally available and prevents intracellular vesicle transport along microtubules, a part of the cytoskeleton. It binds to the colchicine binding site of the alpha and beta tubulin subunits of microtubules and crosslinks the microtubules, thereby inhibiting the polymerization of microtubules in tumor cells and tumor blood vessel endothelial cells.[6] This blocks the formation of the mitotic spindle apparatus and leads to cell cycle arrest in the G2/M phase. As a result, this drug disrupts the formation of blood vessels in the tumor and blood flow in the tumor, depriving the tumor cells of nutrients and triggering apoptosis. In addition, since microtubules play an important role in intracellular transport, inhibition of their polymerization can interfere with the transport of the androgen receptor (AR) into the cell nucleus as well as the transport of a virus inside the cell. This can decrease viral replication and assembly of the virus. Inhibition of tubulin polymerization can also inhibit the release of pro-inflammatory cytokines and disrupt the activities of inflammatory cells.
Sabizabulin is not a substrate of P-glycoprotein (Pgp), an efflux pump that, when overexpressed, can confer resistance to taxanes.
Research
COVID-19 therapy
In a phase III study on the treatment of severe courses of COVID-19,[3][7] sabizabulin reduced mortality by 55% according to the manufacturer.[8] Because of the high efficacy, the test phase was stopped prematurely so that the drug no longer had to be withheld from the placebo control group.[9][10]
References
- "Substance Name: Sabizabulin". ChemIDplus. Retrieved 1 May 2022.
- "Sabizabulin for COVID-19". Veru Inc. 14 January 2022. Retrieved 1 May 2022.
- "VERU-111 in the Treatment of SARS-Cov-2 Infection by Assessing Its Effect on the Proportion of Patients Who Die on Study". ClinicalTrials.gov (Press release). 13 April 2021. Retrieved 1 May 2022.
- Markowski MC, Tutrone R, Pieczonka C, Barnette KG, Getzenberg RH, Rodriguez D, et al. (April 2022). "A Phase 1b/2 Study of Sabizabulin, a Novel Oral Cytoskeleton Disruptor, in Men With Metastatic Castration-Resistant Prostate Cancer with Progression on an Androgen Receptor Targeting Agent". Clinical Cancer Research. doi:10.1158/1078-0432.CCR-22-0162. PMID 35416959.
- rme (13 April 2022). "COVID-19: Krebsmittel Sabizabulin halbiert Sterberate bei schweren Erkrankungen". aerzteblatt.de. Retrieved 14 April 2022.
- "Sabizabulin (Code C158517)". NCI Thesaurus. Retrieved 14 April 2022.
- "Veru Enrolls First Patient in Phase 3 Clinical Trial of Sabizabulin (VERU-111) in High Risk Hospitalized COVID-19 Patients". Veru Inc. 19 May 2021. Retrieved 1 May 2022.
- "Veru's Novel COVID-19 Drug Candidate Reduces Deaths by 55% in Hospitalized Patients in Interim Analysis of Phase 3 Study; Independent Data Monitoring Committee Halts Study Early for Overwhelming Efficacy". Veru Inc. (Press release). 11 April 2022. Retrieved 30 April 2022.
- Rabin, Roni (11 April 2022). "New Drug Slashed Deaths Among Patients With Severe Covid, Maker Claims". The New York Times. Retrieved 21 April 2022.
- "Veru Announces Oral Late-Breaking Presentation of Phase 2 Data of Sabizabulin for the Treatment of Hospitalized Severe COVID-19 Patients at High Risk for Acute Respiratory Distress Syndrome at the 32nd European Congress of Clinical Microbiology & Infectious Diseases" (Press release). Veru Inc. 25 April 2022. Retrieved 30 April 2022 – via GlobeNewswire.
Further reading
- Deng S, Krutilina RI, Wang Q, Lin Z, Parke DN, Playa HC, et al. (February 2020). "An Orally Available Tubulin Inhibitor, VERU-111, Suppresses Triple-Negative Breast Cancer Tumor Growth and Metastasis and Bypasses Taxane Resistance". Mol Cancer Ther. 19 (2): 348–363. doi:10.1158/1535-7163.MCT-19-0536. PMID 31645441.