Photocatalytic concrete
Photo-catalytic concrete is a formulation of concrete used as pavers and other structural concrete that includes titanium dioxide (TiO2) as an admixture or superficial layer. Titanium dioxide is a heterogeneous photocatalyst that uses sunlight and moisture to absorb and render oxides of nitrogen (NO and NO2) into nitrate ions (NO3−), which are then either washed away by rain or soaked into the concrete to form stable compounds.[1]
Mechanism
When titanium dioxide is exposed to ultraviolet radiation from sunlight, it absorbs the radiation and electron excitation occurs. The following reactions then occur on the surface of the titanium dioxide crystals:
Photolysis of water:
- H2O → H+ + OH (hydroxyl radical) + e−
- O2 + e− → O2− (a superoxide ion)
The overall reaction is therefore:
- H2O + O2 → H+ + O2− + OH
The hydroxyl radical is a powerful oxidizing agent and can oxidize nitrogen dioxide to nitrate ions:
- NO2 + OH → H+ + NO3−
The superoxide ion is also able to form nitrate ions from nitrogen monoxide:
- NO + O2− → NO3−
The oxidation of NOx to nitrate ions occurs very slowly under normal atmospheric conditions because of the low concentrations of the reactions. The photochemical oxidation with the aid of titanium dioxide is much faster because of the energy absorbed by the coating on the block and also because the reactants are held together on the surface of the block. The reaction using titanium dioxide shows a greater oxidizing power than most other metal-based catalysts.
Photo-catalytic blocks have replaced ordinary paving in around 30 towns in Japan, originally having been tested in Osaka in 1997 and have been used in the City of Westminster (London). The aim of these blocks is to reduce atmospheric pollution levels and therefore lower the amount of photochemical smog.
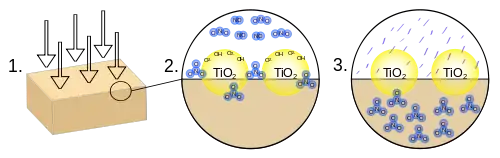
- Ultraviolet radiation is absorbed by the titanium dioxide, which causes the photolysis of water into superoxide ions and hydroxyl radicals.
- Nitrogen oxides react with the superoxide ions and the hydroxyl radicals to form nitrate ions.
- The nitrate ions are absorbed into the block and form stable compounds.
References
- Sikkema, Joel K (2003). "Photocatalytic degradation of NOx by concrete pavement containing TiO2". Iowa State University. Retrieved 14 November 2020.