Mavacamten
Mavacamten, sold under the brand name Camzyos, is a medication used to treat obstructive hypertrophic cardiomyopathy.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Camzyos |
| Other names | MYK-461 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Cardiac myosin inhibitor |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
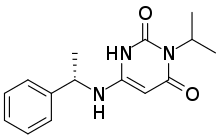
| Formula | C15H19N3O2 |
| Molar mass | 273.336 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mavacamten is a cardiac myosin inhibitor.[1] It was developed by the MyoKardia subsidiary of Bristol Myers Squibb.[2]
Mavacamten was approved for medical use in the United States in April 2022.[1][3]
Medical uses
Mavacamten is indicated for the treatment of adults with symptomatic New York Heart Association class II-III obstructive hypertrophic cardiomyopathy to improve functional capacity and symptoms.[1]
History
Mavacamten was granted orphan drug designation by the U.S. Food and Drug Administration (FDA).[4]
Society and culture
Names
Mavacamten is the international nonproprietary name (INN).[5]
References
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214998s000lbl.pdf
- "Bristol Myers Squibb Completes Acquisition of MyoKardia, Strengthening Company's Leading Cardiovascular Franchise". Business Wire. 17 November 2020. Retrieved 29 April 2022.
- "U.S. Food and Drug Administration Approves Camzyos (mavacamten) for the Treatment of Adults With Symptomatic New York Heart Association Class II-III Obstructive Hypertrophic Cardiomyopathy (HCM) to Improve Functional Capacity and Symptoms" (Press release). Bristol Myers Squibb. 28 April 2022. Retrieved 29 April 2022 – via Business Wire.
- "Mavacamten Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 27 April 2016. Retrieved 29 April 2022.
- World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78". WHO Drug Information. 31 (3). hdl:10665/330961.
Further reading
- Xie J, Wang Y, Xu Y, Fine JT, Lam J, Garrison LP (2022). "Assessing health-related quality-of-life in patients with symptomatic obstructive hypertrophic cardiomyopathy: EQ-5D-based utilities in the EXPLORER-HCM trial". Journal of Medical Economics. 25 (1): 51–58. doi:10.1080/13696998.2021.2011301. PMID 34907813.
External links
- "Mavacamten". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT03470545 for "Clinical Study to Evaluate Mavacamten (MYK-461) in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy (EXPLORER-HCM)" at ClinicalTrials.gov
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.