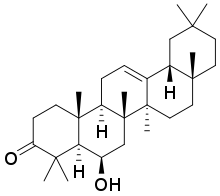
Daturaolone
Daturaolone is a triterpene found in Datura species such as Datura stramonium[1] and Datura innoxia.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(4aR,5R,6aR,6bS,8aR,12aR,14aR,14bR)-5-Hydroxy-4,4,6a,6b,8a,14b-hexamethyl-1,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydropicen-3(2H)-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H48O2 | |
| Molar mass | 440.712 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
History
It was isolated for the first time from Solanum arundo.[3]
Research
It has shown to exhibit dose-dependent anti-inflammatory results in in-vivo models of inflammation by inhibiting COX-1 as well as having potent α-glucosidase and β-secretase inhibitory activity[4] however further studies are needed to better clarify its effect on humans.[5]
It may have antipyretic, muscle relaxant properties and decrease gastrointestinal motility although it is only evaluated in animals. [6]
See also
References
- Li J, Lin B, Wang G, Gao H, Qin M (February 2012). "[Chemical constituents of Datura stramonium seeds]". Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica (in Chinese). 37 (3): 319–22. PMID 22568232.
- Kocór M, Pyrek JS, Atal CK, Bedi KL, Sharma BR (October 1973). "Triterpenes of Datura innoxia Mill. Structure of daturadiol and daturaolone". The Journal of Organic Chemistry. 38 (21): 3685–8. doi:10.1021/jo00961a005. PMID 4745874.
- Grace MH, Saleh MM (August 1996). "Hepato-protective effect of daturaolone isolated from Solanum arundo". Die Pharmazie. 51 (8): 593–5. PMID 8794471.
- Bawazeer, Saud; Rauf, Abdur; Bawazeer, Sami (2020). "Potent In Vitro α-Glucosidase and β-Secretase Inhibition of Amyrin-Type Triterpenoid Isolated from Datura metel Linnaeus (Angel's Trumpet) Fruits". BioMed Research International. 2020: 1–5. doi:10.1155/2020/8530165. ISSN 2314-6133. PMC 7468596.
- Rauf A, Maione F, Uddin G, Raza M, Siddiqui BS, Muhammad N, Shah SU, Khan H, De Feo V, Mascolo N (2016). "Biological Evaluation and Docking Analysis of Daturaolone as Potential Cyclooxygenase Inhibitor". Evidence-Based Complementary and Alternative Medicine. 2016: 4098686. doi:10.1155/2016/4098686. PMC 4793090. PMID 27042189.
- Bawazeer S, Rauf A, Bawazeer S (2020). "Gastrointestinal Motility, Muscle Relaxation, Antipyretic and Acute Toxicity Screening of Amyrin Type Triterpenoid (Daturaolone) Isolated From Datura metel Linnaeus (Angel's Trumpet) Fruits". Frontiers in Pharmacology. 11: 544794. doi:10.3389/fphar.2020.544794. PMC 7546419. PMID 33101017.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.