Cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more rings of carbon atoms. It is formed by cracking cyclohexane in the presence of alumina at a high temperature and pressure.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclopentane | |||
| Other names
pentamethylene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.470 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H10 | |||
| Molar mass | 70.1 g/mol | ||
| Appearance | clear, colorless liquid | ||
| Odor | mild, sweet | ||
| Density | 0.751 g/cm3 | ||
| Melting point | −93.9 °C (−137.0 °F; 179.2 K) | ||
| Boiling point | 49.2 °C (120.6 °F; 322.3 K) | ||
| 156 mg·l−1 (25 °C)[1] | |||
| Solubility | soluble in ethanol, acetone, ether | ||
| Vapor pressure | 45 kPa (20 °C) [2] | ||
| Acidity (pKa) | ~45 | ||
| -59.18·10−6 cm3/mol | |||
Refractive index (nD) |
1.4065 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Flammable[3] | ||
| NFPA 704 (fire diamond) | |||
| Flash point | −37.2 °C (−35.0 °F; 236.0 K) | ||
| 361 °C (682 °F; 634 K) | |||
| Explosive limits | 1.1%-8.7%[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[3] | ||
REL (Recommended) |
TWA 600 ppm (1720 mg/m3)[3] | ||
IDLH (Immediate danger) |
N.D.[3] | ||
| Related compounds | |||
Related compounds |
cyclopropane, cyclobutane, cyclohexane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
It was first prepared in 1893 by the German chemist Johannes Wislicenus.[4]
Industrial usage
Cyclopentane is used in the manufacture of synthetic resins and rubber adhesives and also as a blowing agent in the manufacture of polyurethane insulating foam, and is found in many domestic appliances such as refrigerators and freezers, replacing alternatives such as CFC-11 and HCFC-141b.[5]
Multiply alkylated cyclopentane (MAC) lubricants have low volatility and are used in some specialty applications.[6]
Formulation of cycloalkanes
Cycloalkanes can be formulated via a process known as catalytic reforming.
For example, 2-methylbutane can be reformed into cyclopentane, by use of a platinum catalyst. This is particularly well known in automobiles, as branched alkanes will burn much more readily.
Fluorinated versions
This hydrocarbon has also be fluorinated into compounds such as heptafluorocyclopentane[7] and octafluorocyclopentane,[8] which are used industrially.
Conformations
In a regular pentagon the angles at the vertices are all 108°, slightly less than the bond angle in perfectly tetrahedrally bonded carbon, which is about 109.47°. But cyclopentane is not planar in its normal conformations. It puckers in order to increase the distances between the hydrogen atoms (something which does not happen in the planar cyclopentadienyl anion C5H−5 because it doesn't have as many hydrogen atoms). This means that the average C-C-C angle is less than 108°. There are two conformations that give local minima of the energy, the "envelope" and the "half-chair". The envelope has mirror symmetry (Cs), while the half chair has two-fold rotational symmetry (C2). In both cases the symmetry implies that there are two pairs of equal C-C-C angles and one C-C-C angle that has no pair. In fact for cyclopentane, unlike for cyclohexane (C6H12, see cyclohexane conformation) and higher cycloalkanes, it is not possible geometrically for all the angles and bond lengths to be equal except if it is in the form of a flat regular pentagon.
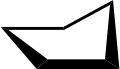 Envelope.
Envelope. 3D envelope.
3D envelope.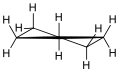 Half-chair.
Half-chair.
References
- Record of cyclopentane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 28 February 2015.
- "ICSC 0353 - CYCLOPENTANE".
- NIOSH Pocket Guide to Chemical Hazards. "#0171". National Institute for Occupational Safety and Health (NIOSH).
- J. Wislicenus and W. Hentschel (1893) "Der Pentamethenylalkohol und seine Derivate" (Cyclopentanol and its derivatives), Annalen der Chemie, 275 : 322-330; see especially pages 327-330. Wislicenus prepared cyclopentane from cyclopentanone ("Ketopentamethen"), which is prepared by heating calcium adipate.
- Greenpeace - Appliance Insulation Archived 2008-10-30 at the Wayback Machine
- Pennzane - lubrication technology
- Zhang, Chengping; Qing, Feiyao; Quan, Hengdao; Sekiya, Akira (January 2016). "Synthesis of 1,1,2,2,3,3,4-heptafluorocyclopentane as a new generation of green solvent". Journal of Fluorine Chemistry. 181: 11–16. doi:10.1016/j.jfluchem.2015.10.012.
- Parthiban, Anbanandam; Ranganathan, Krishnan (November 2016). "Octafluorocyclopentene – A versatile tetrafunctional monomer for making tunable, high surface area, microporous ladder polymers". Journal of Fluorine Chemistry. 191: 70–76. doi:10.1016/j.jfluchem.2016.09.013.
External links
 Media related to Cyclopentane at Wikimedia Commons
Media related to Cyclopentane at Wikimedia Commons