Castor oil glycidyl ether
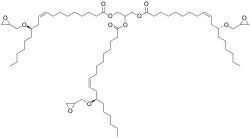
Castor oil glycidyl ether is a liquid organic chemical in the glycidyl ether family. It is sometimes called castor oil triglycidyl ether. It has the theoretical formula C66H116O12 and the CAS number 14228-73-0.[2] The IUPAC name is 2,3-bis[[(E)-12-(oxiran-2-ylmethoxy)octadec-9-enoyl]oxy]propyl (E)-12-(oxiran-2-ylmethoxy)octadec-9-enoate.[3] A key use is acting as a modifier for epoxy resins as a reactive diluent that adds flexibility and improved mechanical properties.[4]
 | |
| Names | |
|---|---|
| IUPAC name
2,3-bis[12-(oxiran-2-ylmethoxy)octadec-9-enoyloxy]propyl 12-(oxiran-2-ylmethoxy)octadec-9-enoate | |
| Identifiers | |
3D model (JSmol) |
|
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C66H16O12 | |
| Molar mass | 1101.6 g/mol |
| Hazards | |
| GHS labelling:[1] | |
 | |
| Warning | |
| H315, H317 | |
| P261, P264, P264+P265, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P333+P313, P337+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Manufacture
It is made by glycidation of castor oil which is a vegetable oil. Castor oil and epichlorohydrin are reacted in the presence of a Lewis acid catalyst to form halohydrin: each hydroxyl group of the triol reacts with an epoxide on epichlorohydrin. This process is followed neutralizing the catalyst with a small amount of sodium hydroxide and then adding a large excess of epichlorohydrin as solvent. To re-form the epoxide rings in a dehydrochlorination reaction, solid sodium hydroxide flake is used rather than a solution. On completion the epichlorohydrin is recovered and the product cleaned up.[5] One of the quality control tests would involve measuring the epoxy value by determination of the epoxy equivalent weight.
Uses
A 2018 study concluded that its use as a flexiblizing agent as well as an epoxy diluent has application in the aviation field.[6] Patent applications show it is also used as a co-reactant-surfactant in herbicide production.[7] As the molecule has 3 oxirane functionalities, a key use is modifying and reducing the viscosity of epoxy resins.[8][9] These reactive diluent modified epoxy resins may then be further formulated into CASE applications: coatings,[10] adhesives,[11] sealants,[12] elastomers. It is also used in composite production.[13] It produces epoxy coatings with high impact resistance.[14] Polymer systems with shape memory may also be produced with this particular molecule.[15] Production of biocompatible materials is also possible and the material is often classed as a renewable resource.[16][17] It has also found use in oil well petroleum recovery.[18]
References
- "Homopolymer of glyceryl triester with 12-glycidyl-9-octadecenoic acid". pubchem.ncbi.nlm.nih.gov. Retrieved 20 April 2022.
- "CASTOR OIL GLYCIDYL ETHER | 74398-71-3". www.chemicalbook.com. Retrieved 2022-04-18.
- PubChem. "Castor oil glycidyl ether". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-04-19.
- Fu, Qinghe; Tan, Jihuai; Han, Changhao; Zhang, Xiaoxiang; Fu, Bo; Wang, Fang; Zhu, Xinbao (November 2020). "Synthesis and curing properties of castor oil‐based triglycidyl ether epoxy resin". Polymers for Advanced Technologies. 31 (11): 2552–2560. doi:10.1002/pat.4982. ISSN 1042-7147. S2CID 225739893.
- US3351574A, Hicks, Darrel D. & Belanger, William J., "Castor oil polyglycidyl ether", issued 1967-11-07
- Ramon, Eric; Sguazzo, Carmen; Moreira, Pedro M. G. P. (October 2018). "A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector". Aerospace. 5 (4): 110. doi:10.3390/aerospace5040110. ISSN 2226-4310.
- , Bevinakatti, Hanamanthsa & Islam, Mojahedul, "Herbicidal Formulations Comprising Glyphosate and Cote-Based Adjuvants", issued 2019-12-19
- Zarnitz, Charles. "Flexibilizing modifiers" (PDF). CVC Thermosets.
- Monte, Salvatore J. (1998), Pritchard, Geoffrey (ed.), "Diluents and viscosity modifiers for epoxy resins", Plastics Additives: An A-Z reference, Polymer Science and Technology Series, Dordrecht: Springer Netherlands, vol. 1, pp. 211–216, doi:10.1007/978-94-011-5862-6_24, ISBN 978-94-011-5862-6, archived from the original on 2022-04-11, retrieved 2022-03-29
- "US Patent Application for EPOXY RESIN COMPOSITION Patent Application (Application #20170029556 issued February 2, 2017) - Justia Patents Search". patents.justia.com. Archived from the original on 2022-04-11. Retrieved 2022-04-11.
- Hao, Xiu; Fan, Dong-Bin (2018-12-17). "Preparation and characterization of epoxy-crosslinked soy protein adhesive". Journal of Adhesion Science and Technology. 32 (24): 2682–2692. doi:10.1080/01694243.2018.1517488. ISSN 0169-4243. S2CID 105550538.
- "14228-73-0 | CAS DataBase". www.chemicalbook.com. Archived from the original on 2022-04-11. Retrieved 2022-04-11.
- Sathyaraj, S.; Sekar, K. (2021). "Recent Advances in Bio-Based Sustainable Aliphatic and Aromatic Epoxy Resins for Composite Applications". Key Engineering Materials. 882: 121–131. doi:10.4028/www.scientific.net/KEM.882.121. ISSN 1662-9795. S2CID 233301700.
- Finter, Jurgen (July 2006). "Low-temperature impact resistant thermosetting epoxide resin compositions with solid epoxide resins US Patent" (PDF). US Patent office.
- Santiago, David; Guzmán, Dailyn; Ferrando, Francesc; Serra, Àngels; De la Flor, Silvia (March 2020). "Bio-Based Epoxy Shape-Memory Thermosets from Triglycidyl Phloroglucinol". Polymers. 12 (3): 542. doi:10.3390/polym12030542. ISSN 2073-4360. PMC 7182903. PMID 32131508.
- Mashouf Roudsari, Ghodsieh; Mohanty, Amar K.; Misra, Manjusri (2017-11-06). "Green Approaches To Engineer Tough Biobased Epoxies: A Review". ACS Sustainable Chemistry & Engineering. 5 (11): 9528–9541. doi:10.1021/acssuschemeng.7b01422. ISSN 2168-0485.
- Ma, Yufeng; Wang, Rui; Li, Qiaoguang; Li, Mei; Liu, Chengguo; Jia, Puyou (2021-03-24). "Castor oil as a platform for preparing bio-based chemicals and polymer materials". Green Materials: 1–11. doi:10.1680/jgrma.20.00085. ISSN 2049-1220. S2CID 233687152.
- "Use of direct epoxy emulsion for well bore stabilization" (PDF). US Patents. December 2010.
Further reading
- Paul F. Bruins; Polytechnic Institute of Brooklyn (1968). Epoxy resin technology. New York: Interscience Publishers. ISBN 0-470-11390-1. OCLC 182890.
- Flick, Ernest W. (1993). Epoxy resins, curing agents, compounds, and modifiers : an industrial guide. Park Ridge, NJ. ISBN 978-0-8155-1708-5. OCLC 915134542.
- Lee, Henry (1967). Handbook of epoxy resins. Kris Neville ([2nd, expanded work] ed.). New York: McGraw-Hill. ISBN 0-07-036997-6. OCLC 311631322.