Carrelame
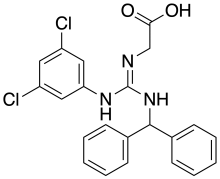
Carrelame is an extremely high potency artificial sweetener of the guanidine class, closely related to lugduname. While Carrelame is roughly 160,000x as sweet as sucrose, lugduname is still somewhat sweeter.[1] It appears safe in pigs.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(Z)-N-{[(3,5-Dichlorophenyl)amino][(diphenylmethyl)amino]methylene}glycine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C22H19Cl2N3O2 | |
| Molar mass | 428.311 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
Additional reading
- O'Brien-Nabors, Lyn (19 April 2016). Alternative Sweeteners. ISBN 9781439846155. Retrieved 13 April 2020.
- "Low-calorie Sweeteners - Google Books". google.com. Retrieved 13 April 2020.
- "Optimising Sweet Taste in Foods - Google Books". google.com. Retrieved 13 April 2020.
References
- Glaser D (2002), "Specialization and phyletic trends of sweetness reception in animals" (PDF), Pure Appl. Chem., 74 (7): 1153–1158, doi:10.1351/pac200274071153, S2CID 97439028
- Nofre, C; Glaser, D; Tinti, JM; Wanner, M (2002). "Gustatory responses of pigs to sixty compounds tasting sweet to humans". Journal of Animal Physiology and Animal Nutrition. 86 (3–4): 90–96. doi:10.1046/j.1439-0396.2002.00361.x. PMID 11972677.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.