Bicapped trigonal prismatic molecular geometry
In chemistry, the bicapped trigonal prismatic molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a biaugmented triangular prism. This shape has C2v symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the dodecahedron.[1][2]
| Bicapped trigonal prismatic molecular geometry | |
|---|---|
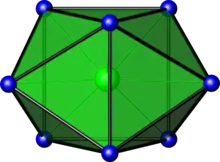 | |
| Examples | ZrF4− 8 |
| Point group | C2v |
| Coordination number | 8 |
It is very similar to the square antiprismatic molecular geometry, and there is some dispute over the specific geometry exhibited by certain molecules. One example of the bicapped trigonal prismatic molecular geometry is the ZrF4−
8 ion.[1]
References
- Jeremy K. Burdett; Roald Hoffmann; Robert C. Fay (1978). "Eight-Coordination". Inorganic Chemistry. 17 (9): 2553–2568. doi:10.1021/ic50187a041.
- Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.