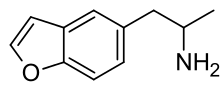
5-APB
5-APB (abbreviation of "5-(2-aminopropyl)benzofuran"; see infobox for the correct IUPAC name) is an empathogenic psychoactive compound of the substituted benzofuran, substituted amphetamine and substituted phenethylamine classes. 5-APB and other compounds are sometimes informally called "Benzofury".
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 1-Benzofuran-5-ylpropan-2-amine |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H13NO |
| Molar mass | 175.231 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
5-APB is commonly found as the succinate and hydrochloride salt. The hydrochloride salt is 10% more potent by mass and doses should be adjusted accordingly.
5-APB has been sold as a designer drug since 2010.[1]
Pharmacology
5-APB is a serotonin–norepinephrine–dopamine reuptake inhibitor with Ki(NET)=180 nmol/L, Ki(DAT)=265 nmol/L and Ki(SERT)=811 nmol/L.[2] It is also a serotonin–norepinephrine–dopamine releasing agent.[3] 5-APB is a potent agonist for the 5-HT2A and 5-HT2B receptors (Ki of 14 nmol/L at 5-HT2B with an efficacy of[2] 0.924). This agonism for 5-HT2B makes it likely that 5-APB would be cardiotoxic with long term use, as seen in other 5-HT2B agonists such as fenfluramine and MDMA. 5-APB is also an agonist of the 5-HT2C receptor.[4]
Detection
A forensic standard of 5-APB is available, and the compound has been posted on the Forendex website of potential drugs of abuse.[5] The US Department of Justice and DEA have also conducted studies concerning the detection of 5-APB.[6]
Effects
Users describe effects as euphoric. Largely, effects reported were similar to that of the drug MDMA but not as strong. Recreational use of 5-APB has been associated with death in combination with other drugs[7][8] and solely as the result of 5-APB.[9]
According to investigative reporting by David Nickles and Lily Kay Ross, a coroner's report identified "complications of 5-APB and other substance abuse" as the cause of death of Jen Rothman, who had been introduced to 5-APB by psychedelic practitioner Jorge Ferrer. The reporters state that the mechanism of death was likely to have been hyponatremia, caused by the increased action of serotonin induced by 5-APB. They note that telltale symptoms of hyponatremia, "Thirst, exhaustion, seizures, and vomiting [were] in Jen’s texts and the police report" pertaining to the incident.[10] Such an effect has been documented for MDMA, which has a similar mechanism of action and subjective effects.[11]
Legality
On March 5, 2014 the UK Home Office announced that 5-APB would be made a class B drug on 10 June 2014 alongside every other benzofuran entactogen and many structurally related drugs.[12]
References
- http://www.emcdda.europa.eu/publications/implementation-reports/2010 EMCDDA–Europol 2010 Annual Report on the implementation of Council Decision 2005/387/JHA
- Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL (January 2013). "Neurochemical profiles of some novel psychoactive substances". European Journal of Pharmacology. 700 (1–3): 147–51. doi:10.1016/j.ejphar.2012.12.006. PMC 3582025. PMID 23261499.
- Rickli A, Kopf S, Hoener MC, Liechti ME (July 2015). "Pharmacological profile of novel psychoactive benzofurans". British Journal of Pharmacology. 172 (13): 3412–25. doi:10.1111/bph.13128. PMC 4500375. PMID 25765500.
- US patent 7045545, Karin Briner et al, "Aminoalkylbenzofurans as serotonin (5-HT(2c)) agonists", published 2000-01-19, issued 2006-16-03
- Southern Association of Forensic Scientists, http://forendex.southernforensic.org/index.php/detail/index/1135 Archived 2014-05-29 at the Wayback Machine
- USDOJ/DEA, http://www.justice.gov/dea/pr/microgram-journals/2011/mj8-2_62-74.pdf
- "UCSD student dies of drug overdose after on-campus music festival". Los Angeles Times. August 20, 2014.
- Dawson P, Opacka-Juffry J, Moffatt JD, Daniju Y, Dutta N, Ramsey J, Davidson C (January 2014). "The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat" (PDF). Progress in Neuro-Psychopharmacology & Biological Psychiatry. 48: 57–63. doi:10.1016/j.pnpbp.2013.08.013. PMID 24012617. S2CID 23400101.
5-APB ... has been implicated in 10 recent drug-related deaths in the UK
- McIntyre IM, Gary RD, Trochta A, Stolberg S, Stabley R (March 2015). "Acute 5-(2-aminopropyl)benzofuran (5-APB) intoxication and fatality: a case report with postmortem concentrations". Journal of Analytical Toxicology. 39 (2): 156–9. doi:10.1093/jat/bku131. PMID 25429871.
- David Nickles and Lily Kay Ross (15 March 2022). "Cover Story, Season 1, Episode 8: Who Am I Fooling" (Podcast). New York Magazine and Psymposia. Retrieved 15 March 2022.
- van Dijken GD, Blom RE, Hené RJ, Boer WH (September 2013). "High incidence of mild hyponatraemia in females using ecstasy at a rave party". Nephrology Dialysis Transplantation. 28 (9): 2277–2283. doi:10.1093/ndt/gft023.
- UK Home Office (2014-03-05). "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Government. Retrieved 2014-03-11.